42 CFR Parts 411, 412, 413, and 489 Medicare Program;
Proposed Changes
to the Hospital Inpatient Prospective Payment Systems and Fiscal Year
2008
Rates; Proposed Rule
F. Hospital-Acquired Conditions,
Including Infections
(If you choose to comment on issues
in this section, please include the
caption "DRGs: Hospital-Acquired
Conditions" at the beginning of your
comment.)
1. General
Medicare's IPPS encourages hospitals
to treat patients efficiently. Hospitals
receive the same DRG payment for stays
that vary in length. In many cases,
complications acquired in the hospital
do not generate higher payments than
the hospital would otherwise receive for
other cases in the same DRG. To this
extent, the IPPS does encourage
hospitals to manage their patients well
and to avoid complications, when
possible. However, complications, such
as infections, acquired in the hospital
can trigger higher payments in two
ways.
- First, the treatment of
complications can increase the cost of
hospital stays enough to generate outlier
payments. However, the outlier
payment methodology requires that
hospitals experience large losses on
outlier cases (for example, in FY 2007,
the fixed-loss amount was $24,485
before a case qualified for outlier
payments, and the hospital then only
received 80 percent of its costs above
the fixed-loss cost threshold).
- Second,
there are about 121 sets of DRGs that
split based on the presence or absence
of a complication or comorbidity (CC).
The CC DRG in each pair would
generate a higher Medicare payment. If
a condition acquired during the
beneficiary's hospital stay is one of the
conditions on the CC list, the result may
be a higher payment to the hospital
under a CC DRG.
Under the proposed
MS-DRGs, there will be 258 sets of
DRGs that are split into 2 or 3 subgroups
based on the presence or absence of a
major CC (MCC) or CC. If a condition
acquired during the beneficiary's
hospital stay is one of the conditions on
the MCC or CC list, the result may be
a higher payment to the hospital under
the MS-DRGs. (See section II.C. of the
FY 2007 IPPS final rule (71 FR 47881)
for a detailed discussion of proposed
DRG reforms.)
2. Legislative Requirement
Section 5001(c) of Pub. L. 109-171
requires the Secretary to select, by
October 1, 2007, at least two conditions
that are
- (a) high cost or high volume or
both,
- (b) result in the assignment of a
case to a DRG that has a higher payment
when present as a secondary diagnosis,
and
- (c) could reasonably have been
prevented through the application of
evidence-based guidelines.
For
discharges occurring on or after October
1, 2008, hospitals will not receive
additional payment for cases in which
one of the selected conditions was not
present on admission. That is, the case
will be paid as though the secondary
diagnosis was not present. Section
5001(c) provides that we can revise the
list of conditions from time to time, as
long as the list contains at least two
conditions. Section 5001(c) also requires
hospitals to submit the secondary
diagnoses that are present at admission
when reporting payment information for
discharges on or after October 1, 2007.
3. Public Input
In the FY 2007 IPPS proposed rule (71
FR 24100), we sought input from the
public about which conditions and
which evidence-based guidelines
should be selected in order to
implement section 5001(c) of Public
Law 109-171. The comments that we
received were summarized in the FY
2007 IPPS final rule (71 FR 48051
through 48053). In that final rule, we
indicated that the next opportunity for
formal public comment would be this
FY 2008 proposed rule and encouraged
the public to comment on our proposal
at that time.
In summary, the majority of the
comments that we received in response
to the FY 2007 IPPS proposed rule
addressed conceptual issues concerning
the selection, measurement, and
prevention of hospital-acquired
infections. Many commenters
encouraged CMS to engage in a
collaborative discussion with relevant
experts in designing, evaluating, and
implementing this section. The
commenters urged CMS to include
individuals with expertise in infection
control and prevention, as well as
representatives from the provider
community, in the discussions.
Many commenters supported the
statutory requirement for hospitals to
submit information regarding secondary
diagnoses present on admission
beginning in FY 2008, and suggested
that it would better enable CMS and
health care providers to more accurately
differentiate between comorbidities and
hospital-acquired complications.
MedPAC, in particular, noted that this
requirement was recommended in its
March 2005 Report to Congress and
indicated that this information is
important to Medicare's value-based
purchasing efforts. Other commenters
cautioned us about potential problems
with relying on secondary diagnosis
codes to identify hospital-acquired
complications, and indicated that
secondary diagnosis codes may be an
inaccurate method for identifying true
hospital-acquired complications.
A number of commenters expressed
concerns about the data coding
requirement for this payment change
and asked for detailed guidance from
CMS to help them identify and
document hospital-acquired
complications. Other commenters
expressed concern that not all hospital-acquired
infections are preventable and
noted that sicker and more complex
patients are at greater risk for hospital-acquired
infections and complications.
Commenters suggested that CMS
include standardized infectionprevention
process measures, in
addition to outcome measures of
hospital-acquired infections.
Some commenters proposed that CMS
expand the scope of the payment
changes beyond the statutory minimum
of two conditions. They noted that the
death, injury, and cost of hospital-acquired
infections are too high to limit
this provision to only two conditions.
Commenters also recommended that
CMS annually select additional
hospital-acquired complications for the
payment change. Conversely, a number
of commenters proposed that CMS
initially begin with limited
demonstrations to test CMS' methodology before nationwide
implementation. One commenter
recommended that CMS include
appropriate consumer protections to
prevent providers from billing patients
for the nonreimbursed costs of the
hospital-acquired complications and to
prevent hospitals from selectively
avoiding patients perceived at risk of
complications.
In addition to the broad conceptual
suggestions, some commenters
recommended specific conditions for
possible inclusion in the payment
changes, which we discuss in detail in
section II.D.4. of this preamble. We also
discuss throughout section II.D. of this
preamble other comments that we have
considered in developing hospital-acquired
conditions that would be
subject to reporting.
4. Collaborative Effort
CMS worked with public health and
infectious disease experts from the
Centers for Disease Control and
Prevention (CDC) to identify a list of
hospital-acquired conditions, including
infections, as required by section
5001(c) of Public Law 109-171. As
previously stated, the selected
conditions must meet the following
three criteria:
- (a) High cost or high
volume or both;
- (b) result in the
assignment of the case to a DRG that has
a higher payment when present as a
secondary diagnosis; and
- (c) could
reasonably have been prevented through
the application of evidence-based
guidelines.
CMS and CDC staff also
collaborated on developing a process for
hospitals to submit a Present on
Admission (POA) indicator with each
secondary condition. The statute
requires the Secretary to begin
collecting this information as of October
1, 2007. The POA indicator is required
in order for us to determine which of
the selected conditions developed
during a hospital stay. The current
electronic format used by hospitals to
obtain this information (ASC X12N 837,
Version 4010) does not provide a field
to obtain the POA information.
We are
in the process of issuing instructions to
require acute care IPPS hospitals to
submit the POA indicator for all
diagnosis codes effective October 1,
2007. The instructions will specify how
hospitals under the IPPS will submit
this information in segment K3 in the
2300 loop, data element K301 on the
ASC X12N 837, Version 4010 claim.
Specific instructions on how to select
the correct POA indicator for a
diagnosis code are included in the ICD-
9-CM Official Guidelines for Coding
and Reporting. These guidelines can be
found at the following Web site:
CMS and CDC staff also received
input from a number of groups and
organizations on hospital-acquired
conditions, including infections. Many
of these groups and organizations
recommended the selection of
conditions mentioned in the FY 2007
IPPS final rule, including the following
because of the high cost or high volume
(frequency) of the condition, or both,
and because in some cases preventable
guidelines already exist:
- Surgical site infections. The groups
and organizations stated that there were
evidence-based measures to prevent the
occurrence of these infections which are
currently measured and reported as part
of the Surgical Care Improvement
Program (SCIP).
- Ventilator-associated pneumonias.
The groups and organizations pointed
out that these conditions are currently
measured and reported through SCIP.
However, other organizations counseled
against selecting these conditions
because they believed it was difficult to
obtain good definitions and that it was
not always clear which ones are
hospital-acquired. Catheter associated bloodstream
infections.
- Pressure ulcers, as an alternative to
hospital-acquired infections. The groups
and organizations pointed out that the
specific language in section 5001(c) of
Public Law 109-171 mentions hospital-acquired
conditions; therefore, the
language does not restrict the Secretary
to the selection of infections.
- Hospital falls, as an alternative to
hospital-acquired infections. The injury
prevention groups included this
condition among a group referred to as
"serious preventable events," also
commonly referred to as "never events"
or "serious reportable events." A serious
preventable event is defined as a
condition which should not occur
during an inpatient stay.
In addition to the aforementioned
conditions, we received other
recommendations for the selection of
hospital-acquired conditions. These
recommendations were also based on
the high cost and the high volume of the
condition, or both, or the fact that
preventable guidelines exist. The
recommendations include:
- Bloodstream infections/septicemia.
Some commenters suggested that we
focus on one specific organism, such as
staph aureus septicemia.
- Pneumonia. Some commenters
recommended the inclusion of a broader
group of pneumonia patients, instead of
restricting cases to ventilator-associated
pneumonias. Some commenters
mentioned that while prevention
guidelines exist for pneumonia, it is not
clear how effective these guidelines may
be in preventing pneumonia.
- Vascular catheter associated
infections. Commenters pointed out that
there are CDC guidelines for these
infections. Other commenters pointed
out that while this condition certainly
deserves focused attention by health
care providers, there is not a clear one
unique ICD-9-CM code that identifies
vascular catheter-associated infections.
Therefore, these commenters suggested
that there would be difficulty separately
identifying these conditions.
- Clostridium difficile-associated
disease (CDAD). Several commenters
identified this condition as a significant
public health issue. Other commenters
pointed out that while prevalence of
this condition is emerging as a public
health problem, there is not currently a
strategy for reasonably preventing these
infections.
- Methicillin-resistant
staphylococcus aureus (MRSA). Several
commenters pointed out that MRSA has
become a very common bacteria
occurring both in and outside the
hospital environment. However, other
organizations pointed out that the code
for MRSA (V09.0, Infection with
microorganism resistant to penicillins
Methicillin-resistant staphylococcus
aureus) is not currently classified as a
CC. Therefore, the commenters stated
that MRSA does not lead to a higher
reimbursement when the code is
reported.
- Serious preventable events. As
stated earlier, some commenters
representing injury prevention groups
suggested including a broader group of
conditions than hospital falls which
should not be expected to occur during
a hospital admission. Hey notes that
these conditions are referred to as
"serious preventable events," and
include events such as the following:
- (a)
Leaving an object in during surgery;
- (b)
operating on the wrong body part or
patient, or performing the wrong
surgery;
- (c) air embolism as a result of
surgery; and
- (d) providing incompatible
blood or blood products.
Other
commenters indicated that serious
preventable events are so rare that they
should not be selected as a hospital
condition that cannot result in a case
being assigned to a higher paying DRG.
5. Criteria for Selection of the Hospital-
Acquired Conditions
CMS and CDC staff greatly appreciate
the many comments and suggestions
offered by organizations and groups that
were interested in providing input into the selection of the initial
hospital-acquired
conditions.
CMS and CDC staff evaluated each
recommended condition under the three
criteria established by section
1886(d)(4)(D)(iv) of the Act. In order to
meet the higher payment criterion, the
condition selected must have an ICD-9-
CM diagnosis code that clearly
identifies the condition and is classified
as a CC, or as an MCC as proposed for
the MS-DRGs in this proposed rule.
Some conditions recommended for
inclusion among the initial hospital-acquired
conditions did not have codes
that clearly identified the conditions.
Because there has not been national
reporting of a POA indicator for each
diagnosis, there is no Medicare data to
determine the incidence of the reported
secondary diagnoses occurring after
admission. To the extent possible, we
used information from the CDC on the
incidence of these conditions. CDC's
data reflect the incidence of hospital-acquired
conditions in 2002. We also
examined FY 2006 Medicare data on the
frequency that these conditions were
reported as secondary diagnoses. We
developed the following criteria to assist
in our analysis of the conditions. The
conditions described were those
recommended for inclusion in the
initial hospital-acquired infection
provision.
Coding - Under section
1886(d)(4)(D)(ii)(I) of the Act, a
discharge is subject to the payment
adjustment if "the discharge includes a
condition identified by a diagnosis
code" selected by the Secretary under
section 1886(d)(4)(D)(iv) of the Act. We
only selected conditions that have (or
could have) a unique ICD-9-CM code
that clearly describes the condition.
Some conditions recommended by the
commenters would require the use of
two or more ICD-9-CM codes to clearly
identify the conditions. Although we
did not exclude these conditions from
further consideration, the need to utilize
multiple ICD-9-CM codes to identify
them may present operational issues.
For instance, below we describe in
detail the complexities associated with
selecting septicemia as a hospital-acquired
condition that would be
subject to section 5001(c) of the DRA. In
some cases, septicemia may be a
reasonably preventable condition with
proper hospital care. However, in other
cases, clinicians may argue that the
condition arose from further
development of another infection the
patient did have upon admission and
the septicemia was not preventable. As
we indicate in detail below, there could
be a significant variety of clinical
scenarios and potential coding vignettes to describe situations where
septicemia
occurs. Although we could select
septicemia, we would also have to
identify many exclusions for situations
where the septicemia is not preventable.
The vast number of clinical scenarios
that we would have to account for could
complicate implementation of the
provision.
- Burden (High Cost/High
Volume) - Under section 1886(d)(4)(D)(iv)(I) of the
act, we must select cases that have
conditions that are high cost or high
volume, or both.
- Prevention guidelines -
Under
section 1886(d)(4)(D)(iv)(II) of the Act,
we must select codes that describe
conditions that could reasonably have
been prevented through application of
evidence-based guidelines. We
evaluated whether there is information
available for hospitals to follow to
prevent the condition from occurring.
- CC - Under section
1886(d)(4)(D)(iv)(III) of the Act, we must
select codes that result in assignment of
the case to a DRG that has a higher
payment when the code it present as a
secondary diagnosis. The condition
must be an MCC or a CC that would, in
the absence of this provision, result in
assignment to a higher paying DRG.
- Considerations - We
evaluate each
condition above according to how it
meets the statutory criteria in light of
the potential difficulties that we would
face if the condition were selected.
6. Proposed Selection of Hospital-
Acquired Conditions
We discuss below our analysis of each
of the conditions that were raised as
possible candidates for selection under
section 5001(c) of Pub. L. 109-171
according to the criteria described above
in section II.D.5. of this preamble. We
also discuss any considerations, which
would include any administrative issues
surrounding the selection of a proposed
condition. For example, the condition
may only be able to be identified by
multiple codes, thereby requiring the
development of special GROUPER logic
to also exclude similar or related ICD-
9-CM codes from being classified as a
CC. Similarly, a condition acquired
during a hospital stay may arise from
another condition that the patient had
prior to admission, making it difficult to
determine whether the condition was
reasonably preventable. Following a
discussion of each condition, we
provide a summary table that describes
the extent to which each condition
meets each of the above criteria.
We
present 13 conditions in rank order. In
our view, the conditions listed at the top
of the table best meet the statutory
selection criteria, while the conditions listed lower may meet the
selection
criteria but could present a particular
challenge (that is, they may be
preventable only in some circumstances
but not in others). Therefore, we would
submit that the first conditions listed
should receive the highest consideration
of selection among our initial group of
hospital-acquired conditions. We
encourage comments on whether or not
we have ranked these conditions
appropriately. We also encourage
additional comments on clinical,
coding, and prevention issues that may
affect the conditions selected. While we
have ranked these conditions, there may
be compelling public health reasons for
including conditions that are not at the
top of our list. We ask commenters to
recommend how many and which
conditions should be selected for
implementation on October 1, 2008,
along with justifications for these
selections.
(a) Catheter-Associated Urinary Tract
Infections
Coding
ICD-9-CM code 996.64
(Infection and inflammatory reaction
due to indwelling urinary catheter)
clearly identifies this condition. The
hospital would also report the code for
the specific type of urinary infection.
For instance, when a patient develops a
catheter associated urinary tract
infection during the inpatient stay, the
hospital would report code 996.64 and
599.0 (Urinary tract infection, site not
specified) to clearly identify the
condition. There are also a number of
other more specific urinary tract
infection codes that could also be coded
with code 996.64. These codes are
classified as CCs. If we were to select
catheter-associated urinary tract
infections, we would implement the
decision by not counting code 996.64
and any of the urinary tract infection
codes listed below when both codes are
present and the condition was acquired
after admission. If only code 996.64
were coded on the claim as a secondary
diagnosis, we would not count it as a
CC.
Burden (High Cost/High Volume)
CDC reports that there are 561,667
catheter-associated urinary tract
infections per year. For FY 2006, there
were 11,780 reported cases of Medicare
patients who had a catheter associated
urinary tract infection as a secondary
diagnosis. The cases had average
charges of $40,347 for the entire
hospital stay. According to a study in
the American Journal of Medicine,
catheter-associated urinary tract
infection is the most common
nosocomial infection, accounting for
more than 1 million cases in hospitals and nursing homes nationwide.
(Foxman, B.: "Epidemiology of urinary tract
infections: incidence, morbidity, and economic
costs," The American Journal of Medicine, 113
Suppl. 1A, pp. 5s-13s, 2002.0 )
Approximately 11.3 million women in
the United States had at least one
presumed acute community-acquired
urinary tract infection resulting in
antimicrobial therapy in 1995, with
direct costs estimated at $659 million
and indirect costs totaling $936 million.
Nosocomial urinary tract infection
necessitates one extra hospital day per
patient, or nearly 1 million extra
hospital days per year. It is estimated
that each episode of symptomatic
urinary tract infection adds $676 to a
hospital bill. In total, according to the
study, the estimated annual cost of
nosocomial urinary tract infection in the
United States ranges between $424 and
$451 million.
Prevention guidelines
There are
widely recognized guidelines for the
prevention of catheter-associated
urinary tract infections. Guidelines can
be found at the following Web site:
http://www.cdc.gov/ncidod/dhqp/
gl_catheter_assoc.html.
CC
Codes 996.64 and 599.0 are
classified as CCs in the current CMS DRGs as well as in the proposed
MS-
DRGs.
Considerations
The primary
prevention intervention would be not
using catheters or removing catheters as
soon as possible, both of which are
worthy goals because once catheters are
in place for 3 to 4 days, most clinicians
and infectious disease/infection control
experts do not believe urinary tract
infections are preventable. While there
may be some concern about the
selection of catheter associated urinary
tract infections, it is an important public
health goal to encourage practices that
will reduce urinary tract infections.
Approximately 40 percent of Medicare
beneficiaries have a urinary catheter
during hospitalization based on
Medicare Patient Safety Monitoring
System (MPSMS) data.
As stated above in the Coding section,
this condition is clearly identified
through ICD-9-CM code 996.64. Code
996.64 is classified as a CC. The hospital
would also report the code for the
specific type of urinary infection. For
instance, when a patient develops a
catheter associated urinary tract
infection during the inpatient stay, the
hospital would report codes 996.64 and
599.0 or another more specific code that
clearly identifies the condition. These
codes are classified as CCs under the
current CMS DRGs as well as the
proposed MS-DRGs.
To select catheter one of the hospital-acquired conditions
that would not be counted as a CC, we
would not classify code 996.64 as a CC
if the condition occurred after
admission. Furthermore, we would also
not classify any of the codes listed
below as CCs if present on the claim
with code 996.64 because these
additional codes identify the same
condition. The following codes
represent specific types of urinary
infections. We did not include codes for
conditions that could be considered
chronic urinary infections, such as code
590.00 (Chronic pyelonephritis, without
lesion or renal medullary necrosis).
Chronic conditions may indicate that
the condition was not acquired during
the current stay. We would not count
code 996.64 or any of the following
codes representing acute urinary
infections if they developed after
admission and were coded together on
the same claim.
- 112.2 (Candidiasis of other
urogenital sites)
- 590.10 (Acute pyelonephritis,
without lesion of renal medullary
necrosis)
- 590.11 (Acute pyelonephritis, with
lesion of renal medullary necrosis)
- 590.2 (Renal and perinephric
abscess)
- 590.3 (Pyeloureteritis cystica)
- 590.80 (Pyelonephritis,
unspecified)
- 590.81 (Pyelitis or pyelonephritis
in diseases classified elsewhere)
- 590.9 (Infection of kidney,
unspecified)
- 595.0 (Acute cystitis)
- 595.3 (Trigonitis)
- 595.4 (Cystitis in diseases
classified elsewhere)
- 595.81 (Cystitis cystica)
- 595.89 (Other specified type of
cystitis, other)
- 595.9 (Cystitis, unspecified)
- 597.0 (Urethral abscess)
- 597.80 (Urethritis, unspecified)
- 599.0 (Urinary tract infection, site
not specified)
We believe the condition of catheter-associated
urinary tract infection meets
all of our criteria for selection as one of
the initial hospital-acquired conditions.
We can easily identify the cases with
ICD-9-CM codes. The condition is a CC
under both the current CMS DRGs and
the proposed MS-DRGs that are
discussed earlier in this proposed rule.
The condition meets our burden
criterion with its high cost and high
frequency. There are prevention
guidelines on which the medical
community agrees. Of all 13 conditions
discussed in this proposed rule, we
believe this condition best meets the criteria discussed.
Therefore, we
are
proposing the selection of catheter-associated
urinary tract infections as
one of the initial hospital-acquired
conditions.
We encourage comments on both the
selection of this condition and the
related conditions that we are proposing
to exclude from being counted as CCs.
(b) Pressure Ulcers
Coding
Pressure ulcers are also
referred to as decubitus ulcers. The
following codes clearly identify
pressure ulcers.
- 707.00 (Decubitus ulcer,
unspecified site)
- 707.01 (Decubitus ulcer, elbow)
- 707.02 (Decubitus ulcer, upper
back)
- 707.03 (Decubitus ulcer, lower
back)
- 707.04 (Decubitus ulcer, hip)
- 707.05 (Decubitus ulcer, buttock)
- 707.06 (Decubitus ulcer, ankle)
- 707.07 (Decubitus ulcer, heel)
- 707.09 (Decubitus ulcer, other site)
Burden (High Cost/High Volume)-
This is both a high-cost and highvolume
condition. For FY 2006, there
were 322,946 reported cases of Medicare
patients who had a pressure ulcer as a
secondary diagnosis. These cases had
average charges for the hospital stay of
$40,381.
Prevention guidelines
Prevention
guidelines can be found at the following
Web sites:
CC
Decubitus ulcer codes are
classified as CCs under the current CMS
DRGs. Codes 707.00, 707.01, and 707.09
are CCs under the proposed MS-DRGs.
Codes 707.02 through 707.07 are
considered MCCs under the proposed
MS-DRGs. As discussed earlier, MCCs
result in even larger payments than CCs.
Considerations
Pressure ulcers are
an important hospital-acquired
complication. Prevention guidelines
exist (non-CDC) and can be
implemented by hospitals. Clinicians
may state that some pressure ulcers
present on admission cannot be
identified (skin is not yet broken (Stage
I) but damage to tissue is already done
and skin will eventually break down.
However, by selecting this condition,
we would provide hospitals the
incentive to perform careful
examination of the skin of patients on
admission to identify decubitus ulcers.
If the condition is present on admission,
the provision will not apply. We are
proposing to include pressure ulcers as
one of our initial hospital-acquired
conditions. This condition can be clearly identified through ICD-9-CM
codes. These codes are classified as a CC
under the current CMS DRGs and as a
CC or MCC under the proposed MS-
DRGs. Pressure ulcers meet the burden
criteria because they are both high cost
and high frequency cases. There are
clear prevention guidelines. While there
is some question as to whether all cases
with developing pressure ulcers can be
identified on admission, we believe the
selection of this condition will result in
a closer examination of the patient's
skin on admission. This will result in
better quality of care. We welcome
comments on the proposed inclusion of
this condition.
Serious Preventable Events
Serious preventable events are events
that should not occur in health care.
The injury prevention community has
developed information on serious
preventable events. CMS reviewed the
list of serious preventable events and
identified those events for which there
was an ICD-9-CM code that would
assist in identifying them. We identified
four types of serious preventable events
to include in our evaluation. These
include leaving an object in a patient;
performing the wrong surgery (surgery
on the wrong body part, wrong patient,
or the wrong surgery); air embolism
following surgery; and providing
incompatible blood or blood products.
Three of these serious preventable
events have unique ICD-9-CM codes to
identify them. There is not a clear and
unique code for surgery performed on
the wrong body part, wrong patient, or
the wrong surgery. Each of these events
is discussed separately.
(c) Serious Preventable Event: Object
Left in During Surgery
Coding
Retention of a foreign object
in a patient after surgery is identified
through ICD-9-CM code 998.4 (Foreign
body accidentally left during a
procedure).
Burden (High Cost/High Volume)
For FY 2006, there were 764 cases
reported of Medicare patients who had
an object left in during surgery reported
as a secondary diagnosis. The average
charges for the hospital stay were
$61,962. This is a rare event. Therefore,
it is not high volume. However, an
individual case will likely have high
costs, given that the patient will need
additional surgery to remove the foreign
body. Potential adverse events
stemming from foreign body could
further raise costs for an individual
case.
Prevention guidelines
There are
widely accepted and clear guidelines for
the prevention of this event. Prevention guidelines for avoiding
leaving objects
in during surgery are located at the
following Web site:
This event should
not occur.
CC
This code is a CC under the
current CMS DRGs as well as under the
proposed MS-DRGs.
Considerations
There are no
significant considerations for this
condition. There is a unique ICD-9-CM
code and wide agreement on the
prevention guidelines. We are proposing
to include this condition as one of our
initial hospital-acquired conditions. The
cases can be clearly identified through
an ICD-9-CM. This code is a CC under
both the current CMS DRGs and the
proposed MS-DRGs. There are clear
prevention guidelines. While the cases
may not meet the high frequency
criterion, they do meet the high-cost
criterion. Individual cases can be high
cost. We welcome comments on
including this condition as one of our
initial hospital-acquired conditions
(d) Serious Preventable Event: Air
Embolism
Coding
An air embolism is
identified through ICD-9-CM code
999.1 (Complications of medical care,
NOS, air embolism).
Burden (High Cost/High Volume)
This event is rare. For FY 2006, there
were 45 reported cases of air embolism
for Medicare patients. The average
charges for the hospital stay were
$66,007.
Prevention guidelines
There are
clear prevention guidelines for air
embolisms. This event should not occur.
Serious preventable event guidelines
can be found at the following Web site:
http://www.qualityindicators.ahrq.gov/
psi_download.htm.
CC
This code is a CC under the
current CMS DRGs and is an MCC under
the proposed MS-DRGs.
Considerations
There are no
significant considerations for this
condition. There is a unique ICD-9-CM
code and wide agreement on the
prevention guidelines. In addition, as
stated earlier, the condition is a CC
under the current CMS DRGs and an
MCC under the proposed MS-DRGs.
While the condition is rare, it does meet
the cost burden criterion because
individual cases can be expensive.
Therefore, air embolism is a high-cost
condition because average charges per
case are high. We welcome comments
on the proposal to include this
condition.
(e) Serious Preventable Event: Blood
Incompatibility
Coding
Delivering ABO-incompatible
blood or blood products is identified by
ICM-9-CM code 999.6 (Complications
of medical care, NOS, ABO
incompatibility reaction).
Burden (High Cost/High Volume)
This event is rare. Therefore, it is not
high volume. For FY 2006, there were
33 reported cases of blood
incompatibility among Medicare
patients, with average charges of
$46,492 for the hospital stay. Therefore,
individual cases have high costs.
Prevention guidelines
There are
prevention guidelines for avoiding the
delivery of incompatible blood or blood
products. The event should not occur.
Serious preventable event guidelines
can be found at the following Web site:
CC
This code is a CC under the
current CMS DRGs as well as the
proposed MS-DRGs.
Considerations
There are no
significant considerations for this
condition. There is a unique ICD-9-CM
code which is classified as a CC under
the CMS DRGs as well as the proposed
MS-DRGs. There is wide agreement on
the prevention guidelines. While this
may not be a high-volume condition,
average charges per case are high.
Therefore, we believe this condition is
a high-cost condition and, therefore,
meets our burden criterion. We are
proposing to include this condition as
one of our initial hospital-acquired
conditions.
(f) Staphylococcus Aureus Bloodstream
Infection/Septicemia
Coding
ICD-9-CM Code 038.11
(Staphylococcus aureus septicemia)
identifies this condition. However, the
codes selected to identify septicemia are
somewhat complex. The following ICD-
9-CM codes may also be reported to
identify septicemia:
- 995.91 (Sepsis) and 995.92 ( Severe
sepsis). These codes are reported as
secondary codes and further define
cases with septicemia.
- 998.59 (Other postoperative
infections). This code includes
septicemia that develops
postoperatively.
- 999.3 (Other infection). This code
includes but is not limited to sepsis/
septicemia resulting from infusion,
injection, transfusion, vaccination
(ventilator-associated pneumonia also
included here).
Burden (High Cost/High Volume)
CDC reports that there are 290,000 cases
of staphylococcus aureus infection annually in hospitalized patients of
which approximately 25 percent are
bloodstream infections or sepsis. For FY
2006, there were 29,500 cases of
Medicare patients who had
staphylococcus aureus infection
reported as a secondary diagnosis. The
average charges for the hospital stay
were $82,678. Inpatient staphylococcus
aureus result in an estimated 2.7 million
days in excess length of stay, $9.5
billion in excess charges, and
approximately 12,000 inpatient deaths
per year.
Prevention guidelines
CDC
guidelines are located at the following
Web site:
CC
Codes 038.11, 995.91, 998.59,
and 999.3 are classified as CCs under
the current CMS DRGs and as MCCs
under the proposed MS-DRGs.
Considerations
Preventive health
care associated bloodstream infections/
septicemia that are preventable are
primarily those that are related to a
central venous/vascular catheter, a
surgical procedure (postoperative
sepsis) or those that are secondary to
another preventable infection (for
example, sepsis due to catheterassociated
urinary tract infection).
Otherwise, physicians and other public
health experts may argue whether
septicemia is reasonably preventable.
The septicemia may not be simply a
hospital-acquired infection. It may
simply be a progression of an infection
that occurred prior to admission.
Furthermore, physicians cannot always
tell whether the condition was hospital-acquired.
We examined whether it
might be better to limit the septicemia
cases to a specific organism (for
example, code 038.11 (Staphylococcus
aureus septicemia)). CDC staff
recommended that we focus on
staphylococcus aureus septicemia
because this condition is a significant
public health issue. As stated earlier,
there is a specific code for
staphylococcus aureus septicemia, code
038.11. Therefore, the cases would be
easy to identify. However, as stated
earlier, while this type of septicemia is
identified through code 038.11, coders
may also provide sepsis code 995.91 or
995.92 to more fully describe the
staphylococcus aureus septicemia.
Codes 995.91 and 995.92 are reported as
secondary codes and further define
cases with septicemia. Codes 995.91 and
995.92 are CCs under the current CMS
DRGs and MCCs under the proposed
MS-DRGs.
- 998.59 (Other postoperative
infections). This code includes
septicemia that develops
postoperatively.
- 999.3 (Other infection). This code
includes but is not limited to sepsis/
septicemia resulting from infusion,
injection, transfusion, vaccination
(ventilator-associated pneumonia also
indexed here).
To implement this condition as one of
our initial ones, we would have to
exclude the specific code for
staphylococcus aureus septicemia,
038.11, and the additional septicemia
codes, 995.91, 995.92, 998.59, and
999.3.
We acknowledge that there are
additional issues involved with the
selection of this condition that may
involve developing an exclusion list of
conditions present on admission for
which we would not apply a CC
exclusion to staphylococcus aureus
septicemia. For example, a patient may
come into the hospital with a
staphylococcus aureus infection such as
pneumonia. The pneumonia might
develop into staphylococcus aureus
septicemia during the admission. It may
be appropriate to consider excluding
cases such as those of patients admitted
with staphylococcus aureus pneumonia
that subsequently develop
staphylococcus aureus septicemia from
the provision.
In order to exclude cases
that did not have a staphylococcus
aureus infection prior to admission, we
would have to develop a list of specific
codes that identified all types of
staphylococcus aureus infections such
as code 482.41 (Pneumonia due to
staphylococcus aureus). We likely
would not apply the new provision to
cases of staphylococcus aureus
septicemia if a patient were admitted
with staphylococcus aureus pneumonia.
However, if the patient had other types
of infections, not classified as being
staphylococcus aureus, and then
developed staphylococcus aureus
septicemia during the admission, we
would apply the provision and exclude
the staphylococcus aureus septicemia as
a CC. We were not able to identify any
other specific ICD-9-CM codes that
identify specific infections as being due
to staphylococcus aureus.
Other types of infections, such as
urinary tract infections, would require
the reporting of an additional code,
041.11 (Staphylococcus aureus), to
identify the staphylococcus aureus
infection. This additional coding
presents administrative issues, because
it will not always be clear which
condition code 041.11 (Staphylococcus
aureus) is describing. We do not believe
it would be appropriate to make code
041.11, in combination with other
codes, subject to the hospital-acquired
conditions provision until we better
understand how to address the associated with their selection.
Therefore, we would exclude
staphylococcus aureus septicemia cases
with code 482.41 reported as being
subject to the hospital-acquired
conditions provision. Stated conversely,
we would allow staphylococcus aureus
septicemia to count as a CC if the
patient was admitted with
staphylococcus aureus pneumonia.
We recognize that there may be other
conditions which we should consider
for this type of exclusion. We are
proposing to include staphylococcus
aureus bloodstream infection/
septicemia (code 038.11) as one of our
initial hospital-acquired conditions. We
would also exclude codes 995.91,
998.59, and 999.3 from counting as an
MCC/CC when they are reported with
code 038.11. The condition can be
clearly identified through ICD-9-CM
codes that are classified as CC under the
current CMS DRGs and MCCs under the
proposed MS-DRGs. The condition
meets our burden criterion by being
both high cost and high volume. There
are prevention guidelines which we
acknowledge are subject to some debate
among the medical community.
We also
acknowledge that we would have to
exclude this condition if a patient were
admitted with a staphylococcus aureus
infection of a more limited location,
such as pneumonia. We encourage
commenters to make suggestions on this
issue and to recommend any other
appropriate exclusion for
staphylococcus aureus septicemia. We
encourage comments on the
appropriateness of selecting
staphylococcus aureus septicemia as
one of our proposed initial hospital-acquired
conditions.
(g) Ventilator Associated Pneumonia
(VAP) and Other Types of Pneumonia
Coding
Pneumonia is identified
through the following codes:
- 073.0 (Ornithosis with pneumonia)
- 112.4 (Candidiasis of lung)
- 136.3 (Pneumocystosis)
- 480.0 (Pneumonia due to
adenovirus)
- 480.1 (Pneumonia due to
respiratory syncytial virus)
- 480.2 (Pneumonia due to
parainfluenza virus)
- 480.3 (Pneumonia due to SARSassociated
coronavirus)
- 480.8 (Pneumonia due to other
virus not elsewhere classified)
- 480.9 (Viral pneumonia,
unspecified)
- 481 (Pneumococcal pneumonia
[Streptococcus pneumoniae
pneumonia] 482.0 (Pneumonia due to Klebsiella
pneumoniae)
- 482.1 (Pneumonia due to
Pseudomonas)
- 482.2 (Pneumonia due to
Hemophilus influenzae [H. influenzae])
- 482.30 (Pneumonia due to
Streptococcus, unspecified)
- 482.31 (Pneumonia due to
Streptococcus, Group A)
- 482.32 (Pneumonia due to
Streptococcus, Group B)
- 482.39 (Pneumonia due to other
Streptococcus)
- 482.40 (Pneumonia due to
Staphylococcus, unspecified)
- 482.41 (Pneumonia due to
Staphylococcus aureus)
- 482.49 (Other Staphylococcus
pneumonia)
- 482.81 (Pneumonia due to
Anaerobes)
- 482.82 (Pneumonia due to
Escherichia coli [E. coli])
- 482.83 (Pneumonia due to other
gram-negative bacteria)
- 482.84 (Pneumonia due to
Legionnaires' disease)
- 482.89 (Pneumonia due to other
specified bacteria)
- 482.9 (Bacterial pneumonia
unspecified)
- 483.0 (Pneumonia due to
Mycoplasma pneumoniae)
There is not a unique code that
identifies ventilator associated
pneumonia.
The creation of a code for
ventilator associated pneumonia was
discussed at the September 29, 2006
meeting of the ICD-9-CM Coordination
and Maintenance Committee meeting.
Many issues and concerns were raised
at the meeting concerning the creation
of this proposed new code. It has been
difficult to define ventilator-associated
pneumonia. We plan to continue
working closely with the CDC to
develop a code that can accurately
describe this condition for
implementation in FY 2009.
CDC will
address the creation of a unique code for
this condition at the September 28-29,
2007 ICD-9-CM Coordination and
Maintenance Committee meeting.
While we list 27 pneumonia codes
above, our clinical advisors do not
believe that all of the codes mentioned
could possibly be associated with
ventilator-associated pneumonia.
Our
clinical advisors specifically question
whether the following codes would ever
represent cases of ventilator-associated
pneumonia: 073.0, 480.0, 480.1, 480.2,
480.3, 480.8, 480.9, and 483.0.
Therefore, we have a range of
pneumonia codes, all of which may not
represent cases that could involve
ventilator-associated pneumonia. In
addition, we do not have a specific code that uniquely identifies cases
of
ventilator-associated pneumonia.
Burden (High Cost/High Volume)
CDC reports that there are 250,205
ventilator-associated pneumonias per
year. Because there is not a unique ICD-
9-CM code for ventilator-associated
pneumonia, there is not accurate data
for FY 2006 on the number of Medicare
patients who had this condition as a
secondary diagnosis. However, we did
examine data for FY 2006 on the
number of Medicare patients who listed
pneumonia as a secondary diagnosis.
There were 92,586 cases with a
secondary diagnosis of pneumonia, with
average charges of $88,781. According
to the journal Critical Care Medicine,
patients with ventilator-associated
pneumonia have statistically
significantly longer intensive care
lengths of stay (mean = 6.10 days) than
those who do not (mean = 5.32-6.87
days). In addition, patients who develop
ventilator-associated pneumonia incur,
on average, greater than or equal to
$10,019 in additional hospital costs
compared to those who do not. (Safdar N.: Clinical and Economic
Consequences of Ventilator-Associated Pneumonia:
A Systematic Review, Critical Care Medicine, 2005,
33(10), pp. 2184-2193.)
Therefore, we believe that this is a high volume
condition.
Prevention guidelines
Prevention
guidelines are located at the following
Web site:
However,
it is not clear how effective these
guidelines are in preventing pneumonia.
Ventilator-associated pneumonia may
be particularly difficult to prevent.
CC
All of the pneumonia codes
listed above are CCs under the current
CMS DRGs and under the proposed
MS-DRGs, except for the following
pneumonia codes which are non-CCs:
073.0, 480.0, 480.1, 480.2, 480.3, 480.8,
480.9, 483.0. However, as mentioned
earlier, there is not a unique ICD-9-CM
code for ventilator-associated
pneumonia. Therefore, this condition
does not currently meet the statutory
criteria for being selected.
Considerations
Hospital-acquired
pneumonias, and specifically ventilator
associated pneumonias, are an
important problem. However, based on
our work with the medical community
to develop specific codes for this
condition, we have learned that it is
difficult to define what constitutes
ventilator associated pneumonia.
Although prevention guidelines exist, it
is not clear how effective these are in
preventing pneumonia. Clinicians
cannot always tell which pneumonias
are acquired in a hospital. In addition, as mentioned above, there is
not a
unique code that identifies ventilator-associated
pneumonia.
There are a
number of codes that capture a range of
pneumonia cases. It is not possible to
specifically identify if these pneumonia
cases are ventilator-associated or arose
from other sources.
Because we cannot
identify cases with ventilator-associated
pneumonia and there are questions
about its preventability, we are not
proposing to select this condition as one
of our initial hospital-acquired
conditions. However, we welcome
public comments on how to create an
ICD-9-CM code that identifies
ventilator-associated pneumonia, and
we encourage participation in our
September 28-29, 2007 ICD-9-CM
Coordination and Maintenance
Committee meeting where this issue
will be discussed. We will reevaluate
the selection of this condition in FY
2009.
(h) Vascular Catheter-Associated
Infections
Coding
The code used to identify
vascular catheter associated infections is
ICD-9-CM code 996.62 (Infection due to
other vascular device, implant, and
graft). This code includes infections
associated with all vascular devices,
implants, and grafts. It does not
uniquely identify a vascular catheter
associated infections. Therefore, there is
not a unique ICD-9-CM code for this
infection. CDC and CMS staff requested
that the ICD-9-CM Coordination and
Maintenance Committee discuss the
creation of a unique ICD-9-CM code for
vascular catheter associated infections
because the issue is important for public
health. The proposal to create a new
ICD-9-CM was discussed at the March
22-23, 2007 meeting of the ICD-9-CM
Coordination and Maintenance
Committee. A summary of this meeting
can be found at: http://www.cdc.gov/
nchs/icd9.htm. Coders would also
assign an additional code for the
infection such as septicemia.
Therefore,
a list of specific infection codes would
have to be developed to go along with
code 996.62. If the vascular catheter
associated infection was hospital-acquired,
the DRG logic would have to
be modified so that neither the code for
the vascular catheter associated
infection along with the specific
infection code would count as a CC.
Burden (High Cost/High Volume)
CDC reports that there are 248,678
central line associated bloodstream
infections per year. It appears to be both
high cost and high volume. However,
we were not able to identify Medicare
data on these cases because there is no
existing unique ICD-9-CM code.
Prevention guidelines
CDC
guidelines are located at the following
Web site:
CC
Code 996.62 is a CC under the
current CMS DRGs and the proposed
MS-DRGs. However, as stated earlier,
this code is broader than vascular
catheter-associated infections.
Therefore, there is not a unique ICD-9-
CM code to identify the condition at this
time, and it does not currently meet the
statutory criteria to be selected.
However, as indicated above, we will be
creating a code(s) to identify this
condition and may select it as a
condition under the provision beginning
in FY 2009.
Considerations
There is not yet a
unique ICD-9-CM code to capture this
condition. If one is implemented on
October 1, 2007, we would be able to
specifically identify these cases. Some
patients require long-term indwelling
catheters, which are more prone to
infections. Ideally catheters should be
changed at certain time intervals.
However, circumstances might prevent
such practice (for example, the patient
has a bleeding diathesis). In addition, a
patient may acquire an infection from
another source which can colonize the
catheter. As mentioned earlier, coders
would also assign an additional code for
the infection, such as septicemia.
Therefore, a list of specific infection
codes would have to be developed to go
along with code 996.62. If the vascular
catheter-associated infection was
hospital-acquired, the DRG logic would
have to be modified so that neither the
code for the vascular catheter-associated
infection along with the specific
infection code would count as a CC.
Without a specific code for infections
due to a catheter, it would be difficult
to identify these patients. Given the
current lack of an ICD-9-CM code for
this condition, we are not proposing to
include it as one of our initial hospital-acquired
conditions at this time.
However, we believe it shows merit for
inclusion in future lists of hospital-acquired
conditions once we have
resolved the coding issues and are able
to better identify the condition in the
Medicare data.
We will reevaluate the
selection of this condition in FY 2009.
We encourage comments on this
condition which was identified as an
important public health issue by several
organizations that provided
recommendations on hospital-acquired
conditions. We are particularly
interested in receiving comments on
how we should handle additional
associated infections that might develop
along with the vascular catheterassociated
infection.
(i) Clostridium Difficile-Associated
Disease (CDAD)
Coding
This condition is identified
by ICD-9-CM code 008.45 (Clostridium
difficile).
Burden (High Cost/High Volume)
CDC reports that there are 178,000 cases
per year in U.S. hospitals. For FY 2006,
there were 110,761 reported cases of
Medicare patients with CDAD as a
secondary diagnosis, with average
charges for the hospital stay of $52,464.
Therefore, this is a high-volume
condition.
Prevention guidelines
Prevention
guidelines are not available. Therefore,
we do not believe this condition can
reasonably be prevented through the
application of evidence-based
guidelines.
CC
Code 008.45 is a CC under the
current CMS DRGs and the proposed
MS-DRGs.
Considerations
CDAD is an
emerging problem with significant
public health importance. If found early
CDAD cases can easily be treated.
However, cases not diagnosed early can
be expensive and difficult to treat.
CDAD occurs in patients on a variety of
antibiotic regiments, many of which are
unavoidable, and therefore
preventability is an issue. We are not
proposing to include CDAD as one of
our initial hospital-acquired conditions
at this time, given the lack of prevention
guidelines. We welcome public
comments on CDAD, specifically on its
preventability and whether there is
potential to develop guidelines to
identify it early in the disease process
and/or diminish its incidence. We will
reevaluate the selection of this
condition in FY 2009.
(j) Methicillin-Resistant Staphylococcus
Aureus (MRSA)
Coding
MRSA is identified by ICD-
9-CM code V09.0 (Infection with
microorganisms resistant to penicillins).
One would also assign a code(s) to
describe the exact nature of the
infection.
Burden (High Cost/High Volume)
For FY 2006, there were 95,103 reported
cases of Medicare patients who had
MRSA as a secondary diagnosis. The
average charges for these cases were
$31,088. This condition is a high-cost
and high-volume infection. MRSA has
become a very common bacteria
occurring both in and outside of the
hospital environment.
Prevention guidelines
CDC
guidelines are located at the following
Web site:
CC
Code V09.0 is not a CC under the
current CMS DRGs and the proposed MS-DRGs. The specific infection would
be identified in a code describing the
exact nature of the infection, which may
be a CC.
Considerations
As stated earlier,
preventability may be hard to ascertain
since the bacteria has become so
common both inside and outside the
hospital. There are also considerations
in identifying MRSA infections because
hospitals would report the code for
MRSA along with additional codes that
would describe the exact nature of the
infection. We would have to develop a
list of specific infections that could be
the result of MRSA.
We are not
proposing to include MRSA as one of
our initial hospital-acquired conditions
because the condition is not a CC. We
recognize that associated conditions
may be a CC. We welcome comments on
the proposal not to include this
condition. Should there be support for
including this condition, we request
recommendations on what codes might
be selected to identify the specific types
of infections associated with MRSA.
(k) Surgical Site Infections
Coding
Surgical site infections are
identified by ICD-9-CM code 998.59
(Other postoperative infection). The
code does not tell the exact location or
nature of the postoperative wound
infection. The code includes wound
infections and additional types of
postoperative infections such as
septicemia. The coding guidelines
instruct the coder to add an additional
code to identify the type of infection. To
implement this condition we would
have to remove both code 998.59 and
the specific infection from counting as
a CC if they occurred after the
admission. We would have to develop
an extensive list of possible infections
that would be subject to the provision.
We may also need to recommend the
creation of a series of new ICD-9-CM
codes to identify various types of
surgical site infections, should this
condition merit inclusion among those
that are subject to the proposed
hospital-acquired conditions provision.
Burden (High Cost/High Volume)
CDC reports that there are 290,485
surgical sites infections each year. As
stated earlier, there is not a unique code
for surgical site infection. Therefore, we
examined Medicare data on patients
with any type of postoperative infection.
For FY 2006, there were 38,763 reported
cases of Medicare patients who had a
postoperative infection. These patients
had average charges for the hospital stay
of $79,504. We are unable to determine
how many of these patients had surgical
site infections.
Prevention guidelines
CDC
guidelines are available at the following
Web site:
CC
Code 998.59 is a CC under the
current CMS DRGs and the proposed
MS-DRGs.
Considerations
As mentioned
earlier, code 998.59 is not exclusive to
surgical site infections. It includes other
types of postoperative infections.
Therefore, code 998.59 does not
currently meet the statutory criteria for
being subject to the provision because it
does not uniquely identify surgical site
infections. To identify surgical site
infections, we would need new codes
that provide more detail about the type
of postoperative infection as well as the
site of the infection. In addition, one
would report both code 998.59 as well
a more specific code for the specific
type of infection, making
implementation difficult.
While there
are prevention guidelines, it is not
always possible to identify the specific
types of surgical infections that are
preventable. Therefore, we are not
proposing to select surgical site
infections as one of our proposed
hospital-acquired conditions at this
time. However, we welcome public
comments on whether we can develop
criteria and codes to identify
preventable surgical site infections that
would assist us in reducing their
incidence. We are exploring ways to
identify surgical site infections and will
reevaluate this condition in FY 2009.
(l) Serious Preventable Event: Surgery
on Wrong Body Part, Patient, or Wrong
Surgery
Coding
Surgery performed on the
wrong body part, wrong patient, or the
wrong surgery would be identified by
ICD-9-CM code E876.5 (Performance of
inappropriate operation). This diagnosis
code does not specifically identify
which of these events has occurred.
Burden (High Cost/High Volume)
As
stated earlier, there are not unique ICD-
9-CM codes which capture surgery
performed on the wrong body part or
the wrong patient, or the wrong surgery.
Therefore, we examined Medicare data
on the code for performance of an
inappropriate operation. For FY 2006,
there was one Medicare case reported
with this code, and the patient had
average charges for the hospital stay of
$24,962. This event is rare. Therefore, it
is not high volume. Individual cases
could have high costs. However, we
were unable to determine the impact
with our limited data.
Prevention guidelines
There are
prevention guidelines for performing
the correct surgery on the correct patient or correct patient's
body
part. This event
should not occur.
CC
This code is not a CC under the
current CMS DRGs and the proposed
MS-DRGs. Therefore, it does not meet
the criteria for selection under section
1886(d)(4)(D)(iv) of the Act. However,
Medicare does not pay for performing
surgery on the wrong body part or
patient, or performing the wrong
surgery. These services are not
considered to be reasonable and
necessary and are excluded from
Medicare coverage.
Considerations
There are significant
considerations for the selection of this
condition. There is not a unique ICD-9-
CM code that would describe the nature
of the inappropriate operation. All types
of inappropriate operations are included
in code E876.5. Unlike other conditions,
performance of an inappropriate
operation is not a complication of a
prior medical event that was medically
necessary. Rather, in this case, there was
a needed intervention but it was done
to either the wrong body part or the
wrong patient, or was not the correct
operation. Thus, a service was
completed that was not reasonable and
necessary and Medicare does not pay for
any inpatient service associated with
the wrong surgery. It is not necessary for
us to select this condition because
Medicare does not pay for it under any
circumstances.
(m) Falls
Coding
There is no single code that
shows that a patient has suffered a fall
in the hospital. Codes would be
assigned to identify the nature of any
resulting injury from the fall such as a
fracture, contusion, concussion, etc.
There is a code to indicate that a patient
fell from bed, code E884.4 (Fall from
bed). One would then assign a code that
identifies the external cause of the
injury (the fall from the bed) and an
additional code(s) for any resulting
injury (a fractured bone).
Burden (High Cost/High Volume)
As
stated earlier, there is not a code to
capture all types of falls. Therefore, we
examined Medicare data on the number
of Medicare beneficiaries who fell out of
bed. For FY 2006, there were 2,591
cases reported of Medicare patients who
fell out of bed. These patients had
average charges of the hospital stay of
$24,962. However, depending on the
nature of the injury, costs may vary in
specific cases.
Prevention guidelines
Falls may or
may not be preventable. Serious
preventable event guidelines can be
found at the following Web site:
CC
Code E884.4 is not a CC under
the current CMS DRGs or the proposed
MS-DRGs.
Considerations
There are not clear
codes that identify all types of falls.
Hospitals would also have to use
additional codes for fractures and other
injuries that result from the fall. In
addition, depending on the
circumstances, the falls may or may not
be preventable. We are not proposing
the inclusion of falls as one of our initial
hospital-acquired conditions at this time
because we can only identify a limited
number of these cases, and they are not
classified as a CC. However, we
welcome public comments on how to
develop codes or coding logic that
would allow us to identify injuries that
result from falls in the hospital so that Medicare would not recognize
the
higher costs associated with treating
patients who acquire these conditions in
the hospital. We will reevaluate this
condition in FY 2009.
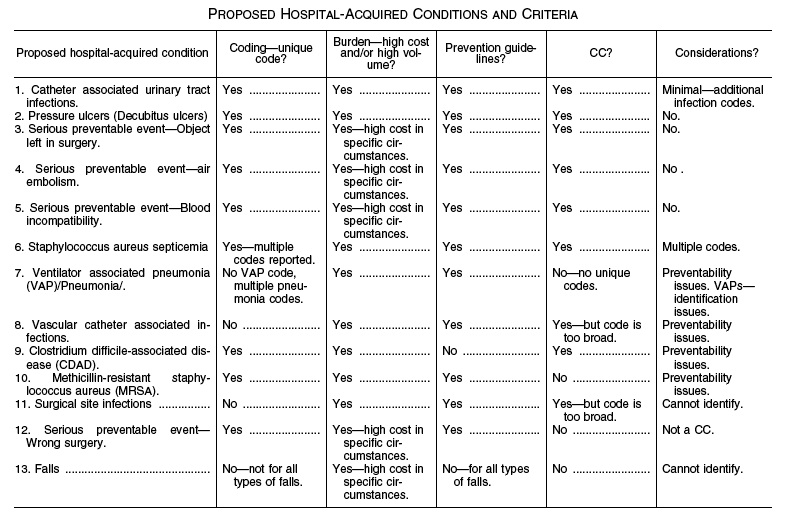
The following table summarizes
whether or not the potential conditions
meet our criteria and if there are
significant considerations with selecting
the particular condition. As mentioned
earlier, we have listed these conditions
in the priority order according to how
well they meet the statutory criteria. As
discussed earlier, we are proposing to
select the first six conditions (catheter
associated urinary tract infections
through Staphylococcus aureus
septicemia) as our initial hospital-acquired
conditions. We would not
include the last seven conditions (ventilator-associated pneumonia
through falls) as initial hospital-acquired
conditions. We welcome
comments on how appropriately we
have evaluated and proposed the
selection of the first six conditions. We
also encourage specific comments on
any additional conditions we should
select for October 1, 2008
implementation. We request
commenters to include a rationale for
selecting any suggested additional
conditions, as well as an analysis of
why each suggested additional
condition meets the criteria under
section 1886(d)(4)(D)(iv) of the Act and
whether there would be coding issues or
other considerations associated with
selecting each condition.
As stated earlier, we are soliciting
comments on the six conditions we
proposed to include among the initial
hospital-acquired conditions. We
welcome any comments on the clinical
aspects of the conditions and on which
conditions should be selected for
implementation on October 1, 2008. We
also solicit comments on any
problematic issues for specific
conditions that may support not
selecting them as one of the initial
conditions. We encourage comments on
how some of the administrative
problems can be overcome if there is
support for a particular condition.
7. Other Issues
Under section 1886(d)(4)(D)(vi) of the
Act, "[a]ny change resulting from the
application of this subparagraph shall
not be taken into account in adjusting
the weighting factors under
subparagraph (C)(i) or in applying
budget neutrality under subparagraph
(C)(iii)." Subparagraph (C)(i) refers to
DRG classifications and relative
weights. Therefore, the statute requires
the Secretary to continue counting the
conditions selected under section
5001(c) of the DRA as MCCs or CCs
when updating the relative weights
annually. Thus, the higher costs associated with a case with a hospital
acquired
MCC or CC will continue to be
assigned to the MCC or CC DRG when
calculating the relative weight but
payment will not be made to the
hospital at one of these higher-paying
DRGs.
Further, subparagraph (C)(iii)
refers to the budget neutrality
calculations that are done so aggregate
payments do not increase as a result of
changes to DRG classifications and
relative weights. Again, the higher costs
associated with the cases that have a
hospital-acquired MCC or CC will be
included in the budget neutrality
calculation but Medicare will make a
lower payment to the hospital for the
specific case that include an MCC or CC.
Thus, to the extent that the provision
applies and cases with an MCC or CC
are assigned to a lower-paying DRG,
section 5001(c) of the DRA will result in
cost savings to the Medicare program.
We note that the provision will only
apply when the selected conditions are
the only MCCs and CCs present on the
claim. Therefore, if a nonselected MCC
or CC is on the claim, the case will
continue to be assigned to the higher
paying MCC or CC DRG, and there will
be no savings to Medicare from the case.
We believe the provision will apply in
a small minority of cases because it is
rare that one of the selected conditions
will be the only MCC or CC present on
the claim. We provide our estimate of
the savings associated with this
provision in the impact section of this
proposed rule.
